Advertisement

Metal parts underwater are subjected to two basic types of corrosion: galvanic corrosion and stray current corrosion. Both can harm your boat, propeller, and motor if not correctly monitored and avoided.
What is Corrosion?
There is nothing mysterious about corrosion. The process metal goes through in changing is slightly complicated, but not especially complex.
To best describe corrosion, let's start with the most common type, rust. We all know rust, but to understand rust, we have to go back to the very beginning. Iron ore has a chemical composition of two iron atoms bonded with three oxygen atoms. As it is mined out of the ground, it's a brownish-red powder useless to us. But by refining, purifying, and smelting, we create iron, which is useful. We can use it as plain iron, or we can process it further and combine it with other elements to get different types of steel.
Let's say the iron is made into hinges for your backyard fence. Everyone knows that if you leave iron out in the rain, it rusts. If it rusts long and badly enough, the metal disappears and you're left with a pile of brownish-red powder-rust or iron oxide, which has the same composition as iron ore.
Here's why. Iron atoms want to return to their normal state as iron ore, iron oxide, or rust. Which are all the same things. That's the state in which iron is most comfortable and most stable. Left alone, it won't turn into anything else. And most metals used in manufactured products want to do the same — return to their natural state.
Electrochemical Reactions
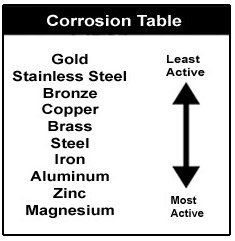
In looking at this chart, you can also see that gold is the least active metal of all metals, however magnesium is the most active, therefore the most likely to protect your boat from currents.
Iron left out in the rain results in a specific kind of corrosion. It's called an electrochemical reaction, meaning there is an electrical change. Here's how that works:
For two iron atoms to really interlock with three oxygen atoms and make iron, they have to share some electrons, which releases a few electrons. Since electricity is just a flow of electrons, those free electrons become a little bit of electricity when the chemical change takes place.
Remember the iron wants to corrode into iron oxide because that is its natural, most stable state. And all it needs for this to take place is oxygen. Water is a supply of oxygen, so iron rusts fastest when it gets wet. You knew that already but now you know why. And that same scenario applies to aluminum and aluminum oxide. Those are the deep, dark secrets of corrosion as they apply to metals. Those are also the basics of an electrochemical reaction, which is known as galvanic corrosion. All galvanic corrosion is an electrical reaction. Not all electrochemical reactions, however, are galvanic corrosion.
Galvanic Corrosion

Galvanic corrosion is an electrochemical reaction between two or more different metals. The metals must be different because one must be more chemically active (or less stable) than the others for a reaction to take place. When we talk about galvanic corrosion, we're talking about electrical exchange. All metals have electrical potential because all atoms have electrons, which have an electrochemical charge.
Galvanic corrosion of the more chemically active metal can occur whenever two or more dissimilar metals that are "grounded" (connected by actually touching each other, or through a wire or metal part) are immersed in a conductive solution (any liquid that can transfer electricity). Anything but pure water is conductive. Saltwater, freshwater with high mineral content, and polluted freshwater are very conductive, and conductivity goes up with water temperature. That's one reason why boats in Florida experience more corrosion than boats in Maine.
The simplest example of galvanic corrosion, and the most applicable, is an aluminum lower unit with a stainless steel propeller. The aluminum is the more chemically active metal (the anode), and the stainless steel is the less chemically active metal (the cathode). Several things happen at the same time:
At the Anode
- Electrons flow from the anode, the metal that is more chemically active (the aluminum drive unit), via the external conducting path to the cathode, the metal that is less chemically active (the stainless steel prop).
- When this happens, the more chemically active metal atoms become ions (an atom with one or more electrons either missing or added) and break away into the water, where they can bond to oxygen ions, with which they can share electrons and produce aluminum oxide. This is the same process iron ions go through when combining with oxygen ions in water to form iron oxide.
- The newly formed aluminum oxide molecules either drift away in the water or settle on the surface of the aluminum. Your lower unit is literally dissolving through galvanic corrosion.
At the Cathode
- Electrons are accepted from the anode; however, they cannot simply accumulate, they react with ions in the electrolyte.
- The resulting hydroxide ion is alkaline, and makes the electrolyte alkaline in the area of the cathode. This detail is especially important for wooden boats, as an alkaline solution will attack cellulose (i.e. wood).
It's important to understand that for each positive metallic ion released at the anode, electrons in the cathode react to form a negative ion in the electrolyte. Electrically the anodic and cathodic reactions must be equivalent. Increases or decreases in the rate of the cathodic reaction will have a corresponding increase or decrease on the anodic reaction. This is a basic fact in understanding and controlling corrosion. This fact can also be demonstrated by the effect of size ratios between anodes and cathodes. If there is a very large anode connected to a small cathode, the anode will corrode very slowly. However, if a very large cathode is connected to a small anode, the anode will corrode very rapidly. Marine drive components have many aluminum parts. If you do not control galvanic corrosion, over time the aluminum will corrode away.
Galvanic corrosion can also occur without any stainless steel components on your boat. For example, you have an aluminum drive unit and an aluminum propeller, but you dock at a pier with steel pilings or a steel seawall, then plug into shore power. The ground wire, which is grounded, connects your aluminum components with the submerged steel because the steel is also grounded. Considering the mass of a seawall or even a single piling, your drive and propeller can sustain serious damage. This damage could be prevented with a galvanic isolator.
What to Look For

Signs of corrosion include blistering paint and the formation of a white powdery substance on the exposed metal areas.
The first sign of galvanic corrosion is paint blistering (starting on sharp edges) below the water line — a white powdery substance forms on the exposed metal areas. As the corrosion continues, the exposed metal areas will become deeply pitted, as the metal is actually eaten away.
Galvanic corrosion of aluminum drive units — or any underwater aluminum on your boat — is accelerated by attaching stainless steel components like propellers, trim planes (if connected to engine ground), and aftermarket steering aids. In doing this, you have introduced a dissimilar metal to which electrons from your drive unit will follow. Another condition that will increase the speed or intensity of galvanic corrosion is the removal or reduction in surface area of sacrificial anodes. But you don't need stainless steel components for galvanic corrosion to take place. Galvanic corrosion continually affects all underwater aluminum, but at a reduced rate when no dissimilar metals are connected to your aluminum parts. When in contact with an electrolyte, most metals form small anodes and cathodes on their surfaces due to such things as alloy segregation, impurities, or cold working.
We have used stainless steel (cathode) and aluminum (anode) in this discussion as an example, however other metals coupled with aluminum also produce galvanic corrosion cells. For example, zinc connected to aluminum will form a corrosion cell, but in this case, the aluminum becomes the cathode and the zinc (anode) corrodes. One of the worst couples with an aluminum drive would be connecting it with copper or a copper alloy (bronze). Another cause of galvanic corrosion is the shore power hookup. When you plug in, you tie your aluminum drive unit to other boats using shore power through the green grounding lead. Your aluminum drive unit is now part of a large galvanic cell (a battery) interconnected with onshore metal that is in the water — as well as other boats — and corrosion may be greatly accelerated.
Stray Current Corrosion
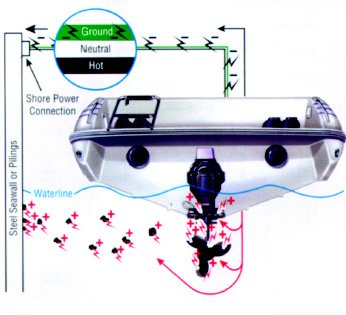
Galvanic corrosion caused by nearby grounded steel structures can occur when you dock your boat and use shore power.
We've discussed what galvanic corrosion can do, using just the electrical potential in metals. Imagine what happens if you add more electricity. That's exactly the basis for stray current corrosion.
Stray current corrosion occurs when metal with an electrical current flowing into it is immersed in water that is grounded (such as in any lake, river, or ocean). The current can leave the metal and flow through the water to ground. This will cause rapid corrosion of the metal at the point where the current leaves. Stray direct current (or battery current) is particularly destructive. Stray current corrosion can cause rapid deterioration of the metal. If the metal in question happens to be an aluminum part like your drive unit, it can be destroyed in a matter of days.
Stray current corrosion is different from galvanic corrosion in that galvanic corrosion is caused by connections between dissimilar metals of your boat's drive components, and utilizes the electrical potential of those dissimilar metals. Electrons flow from one dissimilar metal (the anode) to another dissimilar metal (the cathode). In stray current corrosion, electricity from an outside source flows into your boat's metal components and out through the water for a ground.
For example, your boat may be sitting between a boat leaking DC current and the best ground for that current. Rather than the DC current moving through the water to ground, your boat could provide a path of lower resistance. The DC current could enter a thru-hull fitting, travel through the bonding system, and leave via your drive to the ground. Remember that corrosion occurs at the locations where DC current leaves metal and enters water.
Stray current can come from an outside source either internal or external to your boat. Internal sources involve a short in your boat's wiring system, such as a poorly insulated wire in the bilge, an electrical accessory that may be improperly wired, or a wire with a weak or broken insulation that is intermittently wet.
External sources are almost always related to shore power connections. A boat with internal stray current problems can cause accelerated corrosion to other boats plugged into the same shore power line if they provide better ground. The stray current would be transmitted to other boats through the common ground wire, but can and should be blocked by installing a galvanic isolator.

A much more subtle, but potentially more damaging cause of stray current corrosion can occur without any electrical problems. Suppose you cruise back to the marina after a weekend on the water, and plug into shore power to recharge batteries using your automatic trickle charger. Then you go to work for the week. On Monday, a large steel hulled boat (with scratched and scraped paint) ties up next to your boat. This boat is also plugged into shore power and goes visiting onshore for a few days. A battery has just been formed — the large steel hull and your small aluminum drive connected by the shore power and ground wire. Depending on the proximity, relative sizes, and how long your neighbor is ashore, when you go out the next weekend you may find your drive highly deteriorated. This unfortunate scenario can also be prevented by the installation of a galvanic isolator.
There is greater danger for boats that connect to AC shore power: destructive, low-voltage galvanic currents (DC) passing through the shore power ground wire. Normally, AC is not a corrosion problem, but because the boat, pier, and wire are all connected, or due to a leakage, there can be direct current (DC) also present. This is potentially very damaging and requires additional protection.
Safety regulations require a three-wire cable for carrying shore power aboard any boat, and that one of these leads grounds all electrical and propulsion equipment to shore. This safety procedure reduces the danger of shock, but also connects the underwater metal components on your boat with metal on neighboring boats using shore power, steel piers, and metal objects on shore that extend into the water. This interconnecting of dissimilar metals allows destructive galvanic currents to flow between them. If these currents are allowed to continue, your drive unit will experience severe corrosion damage in a very short time — as little as a few days.
There is a common misconception that you can overprotect your drive by using too many zinc or sacrificial aluminum anodes. This is not true. The corrosion potential of any metal is a voltage that can be measured by a reference electrode. Such measurements in water commonly are made with a silver/silver chloride reference electrode. The corrosion potential of a sacrificial anode is a characteristic value for that metal, and it does matter if you have one piece of the metal or 100 pieces. The corrosion potential stays the same. Of course, 100 anodes would be expensive, heavy, and a considerable drag underwater. Only by increasing the corrosion potential by using a different anode material (such as magnesium in seawater) can you overprotect your drive.
Crevice Corrosion

There is also a form of corrosion that affects many metals, particularly stainless steel, called crevice corrosion. A crevice may be formed under any of the following: deposits (such as silt or sand), plastic washers, fibrous gaskets, or tightly wrapped fishing line. It can also form where moisture can get in and not back out, forming a stagnant zone. Stainless steel is an iron-based alloy containing chrome and nickel. The quality that causes it to be stainless (non-rusting) is its formation of a thin, tightly adhering surface layer of chrome oxide. If this surface is deprived of oxygen, the oxide layer breaks down and the stainless steel will rust just like plain steel. In other words, stainless steel is only stainless when it has access to oxygen. In a crevice where there is moisture depleted of oxygen, stainless steel rusts. The simplest prevention for this condition is to seal out the moisture or clean off any deposits.
Antifouling Paint On Drives
Fouling is a major concern in many situations. Marine animals (barnacles, mussels, etc.) and vegetation can make life miserable for boaters. Antifouling paints are available, but some can affect corrosion protection or even accelerate corrosion.
In the past, tributyltin-(sometimes referred to as TBT or organotin) based antifouling paints controlled fouling and did not cause corrosion problems for aluminum drives. However, environmental concerns and legislation have restricted or prohibited the use of tributyltin paints. Presently, tributyltin-based paints must be applied by a state-licensed repair shop. In the United States and Canada, tributyltin is prohibited for vessels less than 25 meters with an exemption for aluminum hulls, fittings, and drives. If TBT paint can be obtained, it is still recommended for drives.
Galvanic Isolators
Galvanic isolators are solid-state devices that are part of a series connected in line to the boat's green safety ground lead ahead of all grounding connections on the boat. This device functions as a filter, blocking the flow of destructive low voltage galvanic (DC) currents but still maintaining the integrity of the safety grounding circuit.
Inactive Sacrificial Anodes
If the underwater portion of the drive unit shows signs of corrosion, but the sacrificial anodes are not being consumed, the problem may be due to the following:
The sacrificial anodes may not be making good electrical contact with the drive unit. Remove the anode, scrape the mounting surfaces on the part to be protected down to bare metal, and reinstall anodes.
The zinc sacrificial anodes may have a protective coating of a very dense oxide film on their surface (which usually has a charcoal-gray appearance). This condition usually occurs in freshwater, but it can also happen in saltwater areas.
To confirm this condition, test for continuity between the anode and the drive using a multimeter set to "ohms" on the R x 1 scale. If the anode must be scraped with a knife in order to get a conductive reading, the anode is oxidized and should be replaced. Sanding the surface with coarse sandpaper provides a temporary solution, but the oxide will form again.
Some Cautions
Due to the location of the sacrificial trim tab, the drive unit must be kept in the "in" position when the boat is moored. If the drive unit is raised, the trim tab may be out of the water and, therefore, unable to act as a galvanic corrosion inhibitor. (Note from BoatUS editor: it is possible and preferable to place other additional sacrificial zincs on the bracket or elsewhere so that the motor can be raised.)
Do not paint anodes. Painting them will render them inoperative. The anodes will not provide corrosion protection when the boat is removed from the water, therefore the drive unit should be flushed with freshwater to remove saltwater and pollutants prior to storage. For example, dried salt deposits can react with moisture in the air or create a cell and corrode metal.
Do not attempt to use magnesium anodes in saltwater. They will provide overprotection. Over protection will result in a different electrochemical reaction that will create hydrogen on the metal surface of the drive, under the paint. The paint will blister and peel completely off the surface of the overprotected drive.
Corrosion Protection Testing and Troubleshooting
For diagnostic tests, a simple digital volt/ohm meter (multimeter) is necessary. An analog version may be used, but it must be a high-impedance model. Even the most inexpensive digital volt/ohm meter has high impedance.
One of the most helpful methods for determining if corrosion below the waterline is occurring is through the measurement of the hull potential. This is done by immersing a reference electrode, usually silver/silver chloride (a silver wire with a coating of silver chloride) into the water about six inches behind the drive. This electrode is connected to the negative terminal of a digital volt/ohm meter. The positive lead from the meter is attached to the battery ground. With the meter on a two-volt DC scale, the hull potential is displayed. When performing tests, be sure to make sure your battery is fully charged. Also, new boats will usually produce higher readings than normal. This is because the drive unit is being protected by a new finish and new sacrificial anodes. To obtain an accurate diagnosis, the test should be performed after the boat has been used at least one or two weeks. All boats should be moored for at least eight hours before performing the test. This is necessary in order to allow the cathode system and sacrificial anodes to polarize the water molecules in direct contact with the drive. Be careful not to rock the boat excessively while boarding to perform the test, as this will alter the reading. (Note from BoatUS editor: If you are not thoroughly familiar with safety procedures in dealing with electricity on the water, leave this to a qualified professional.)
The first signs of corrosion below the waterline are paint blistering, usually on sharp edges, and the formation of powdery white corrosion material on exposed aluminum surfaces. If the corrosion is allowed to continue, pitting of the aluminum will occur. The chart below may help you determine the cause of the corrosion and the corrective action needed to prevent its continuance.
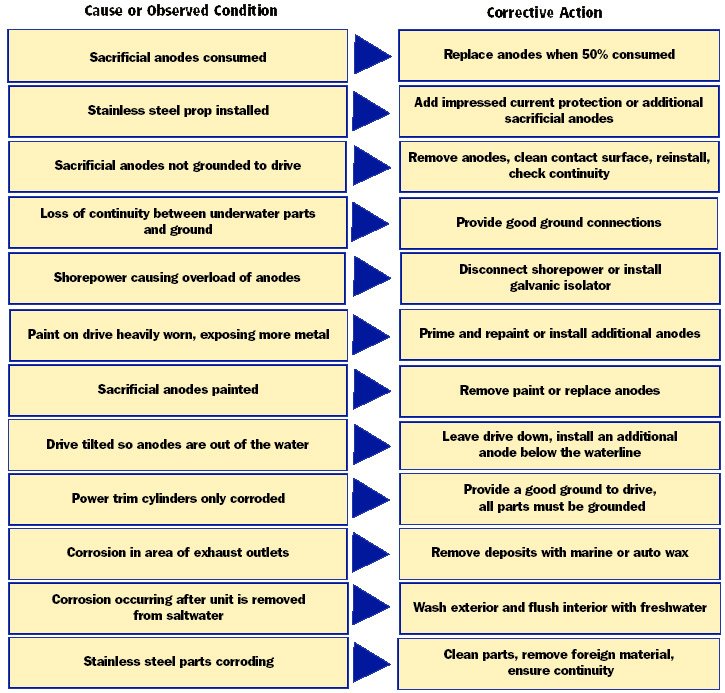
Corrosion Condition Action Chart
